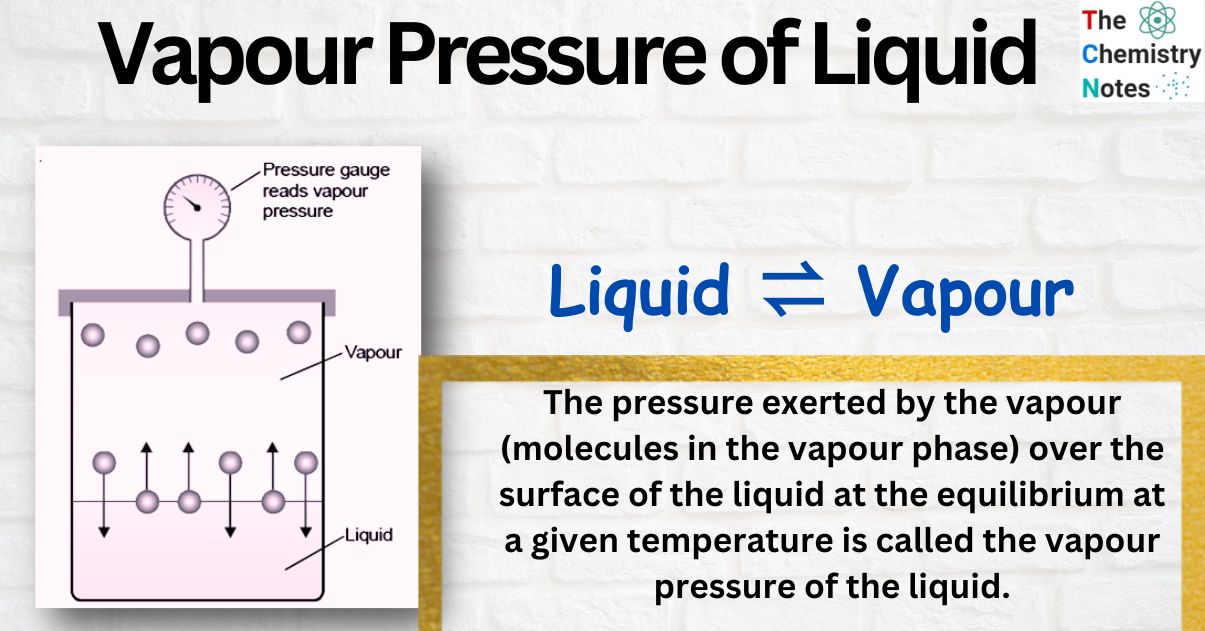
Why Do Volatile Liquids Have High Vapor Pressure . substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container;. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from. Volatility generally decreases with increasing molecular weight. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Basically, the more atoms a molecule has, the more likely it. a volatile substance has a measurable vapor pressure, which is the partial pressure due to the molecules that have entered the gas.
from thechemistrynotes.com
volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container;. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Basically, the more atoms a molecule has, the more likely it. a volatile substance has a measurable vapor pressure, which is the partial pressure due to the molecules that have entered the gas. Volatility generally decreases with increasing molecular weight. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. That is, the pressure of the vapor resulting from. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);
Vapour Pressure of Liquid Definition, Unit, Raoult's Law
Why Do Volatile Liquids Have High Vapor Pressure substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. a volatile substance has a measurable vapor pressure, which is the partial pressure due to the molecules that have entered the gas. Volatility generally decreases with increasing molecular weight. Basically, the more atoms a molecule has, the more likely it. volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container;. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. That is, the pressure of the vapor resulting from.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition, Conditions, Characteristics Why Do Volatile Liquids Have High Vapor Pressure vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. That is, the pressure of the vapor resulting from. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. volatile liquids are liquids with high vapor. Why Do Volatile Liquids Have High Vapor Pressure.
From www.youtube.com
Raoult’s Law For Volatile Solutes (Vapour Pressure Of Liquid Liquid Solution) [Hindi Why Do Volatile Liquids Have High Vapor Pressure a volatile substance has a measurable vapor pressure, which is the partial pressure due to the molecules that have entered the gas. volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container;. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Vapor Pressure and Changes of State PowerPoint Presentation, free download ID9405288 Why Do Volatile Liquids Have High Vapor Pressure the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Basically, the more atoms a molecule has, the more likely it. volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container;. a volatile substance has a measurable vapor pressure, which is the. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Why Do Volatile Liquids Have High Vapor Pressure the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. volatile liquids are liquids with high vapor pressures, which tend to evaporate readily. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 Why Do Volatile Liquids Have High Vapor Pressure the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from. Volatility generally decreases with increasing molecular weight. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed.. Why Do Volatile Liquids Have High Vapor Pressure.
From questions-in.kunduz.com
Two volatile liquids A and B have vapour Physical Chemistry Why Do Volatile Liquids Have High Vapor Pressure vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container;. That is, the pressure of the vapor resulting from. Volatility generally decreases with increasing molecular weight. Basically,. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2735913 Why Do Volatile Liquids Have High Vapor Pressure the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2735913 Why Do Volatile Liquids Have High Vapor Pressure Volatility generally decreases with increasing molecular weight. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. substances with strong intermolecular forces will have lower. Why Do Volatile Liquids Have High Vapor Pressure.
From inspiritvr.com
Vapor Pressure Lowering Study Guide Inspirit Learning Inc Why Do Volatile Liquids Have High Vapor Pressure Volatility generally decreases with increasing molecular weight. That is, the pressure of the vapor resulting from. a volatile substance has a measurable vapor pressure, which is the partial pressure due to the molecules that have entered the gas. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. volatile liquids. Why Do Volatile Liquids Have High Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Why Do Volatile Liquids Have High Vapor Pressure the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Basically, the more atoms a molecule has, the more likely it. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. a volatile substance has a measurable vapor pressure, which is the. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideshare.net
Solutions Powerpoint Why Do Volatile Liquids Have High Vapor Pressure a volatile substance has a measurable vapor pressure, which is the partial pressure due to the molecules that have entered the gas. That is, the pressure of the vapor resulting from. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Basically, the more. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Why Do Volatile Liquids Have High Vapor Pressure Basically, the more atoms a molecule has, the more likely it. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Volatility generally decreases with increasing molecular weight. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 Why Do Volatile Liquids Have High Vapor Pressure Basically, the more atoms a molecule has, the more likely it. That is, the pressure of the vapor resulting from. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. a volatile substance has a measurable vapor pressure, which is the partial pressure due. Why Do Volatile Liquids Have High Vapor Pressure.
From slideplayer.com
Intermolecular forces ppt download Why Do Volatile Liquids Have High Vapor Pressure Basically, the more atoms a molecule has, the more likely it. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. a volatile substance has a measurable vapor pressure, which is the partial pressure due to the molecules that have entered the gas. vapor pressure is the pressure exerted by. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Why Do Volatile Liquids Have High Vapor Pressure a volatile substance has a measurable vapor pressure, which is the partial pressure due to the molecules that have entered the gas. Volatility generally decreases with increasing molecular weight. volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container;. substances with strong intermolecular forces will have lower vapor pressure, because. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT PHASE EQUILIBRIUM PowerPoint Presentation, free download ID6571055 Why Do Volatile Liquids Have High Vapor Pressure vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. That is, the pressure of the vapor resulting from. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Volatility generally decreases with increasing molecular weight.. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT PHASE EQUILIBRIUM PowerPoint Presentation, free download ID9682586 Why Do Volatile Liquids Have High Vapor Pressure That is, the pressure of the vapor resulting from. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. a volatile substance has a measurable vapor. Why Do Volatile Liquids Have High Vapor Pressure.
From www.slideserve.com
PPT Intermolecular Forces, Gases, and Liquids PowerPoint Presentation ID6715060 Why Do Volatile Liquids Have High Vapor Pressure vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic. That is, the pressure of the vapor resulting from. the vapor pressure of a liquid is. Why Do Volatile Liquids Have High Vapor Pressure.